Liquid Crystalline Soft Matter
Surfactants
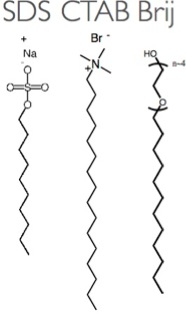
Amphiphilic molecules such as the surfactants (surface active agents) frequently employed in cleaning agents or as stabilizers for colloidal suspensions play a key role in our research. The term amphiphilic means that the molecules have (at least) two moieties with differing preferential environment, usually one side preferring a water surrounding (hydrophilic side) whereas the other prefers an oil-like environment, making it a disliked regime for water (oleophilic or hydrophobic side). Water is indeed the most common solvent when working with surfactants, the reason being the great importance of hydrogen bonding in water. It is easy to design a molecule with a polar head group (ionic or with an oligoethylene glycol unit) that can form hydrogen bonds with water, thus being hydrophilic, connected to a hydrophobic alkyl chain. The contrast within the molecule can induce beautiful self-organization processes when surfactants are dissolved in water, resulting in the formation of micelles and, at higher concentration, lyotropic liquid crystals, both key components of the research in the SNM lab.
 Back to research overview map.
Back to research overview map.