Micelles
If surfactants are present at sufficient concentration in water the hydrophobic effect (the disliking of water of the hydrogen bond-incapable, thus hydrophobic, alkyl chain of the surfactant) will lead to self-organization of the surfactants into closed aggregates, in which the polar head groups arrange outwards towards the water, protecting the hydrophobic alkyl chains from water contact inside the aggregate. These aggregates are the micelles. At small concentration they are often spherical but at higher concentration, or in combination with co-surfactants, they can be disc- or rod-like. If such ansiometric micelles are present at high enough number, they can organize into an orientationally ordered lyotropic liquid crystal phase, a phenomenon that we frequently take advantage of in the ESMP group research.
 Back to research overview.
Back to research overview.Three most recent publications
Isotropic-isotropic phase separation and spinodal decomposition in liquid crystal-solvent mixtures, Catherine G. Reyes, Jörg Baller, Takeaki Araki and Jan P. F. Lagerwall , soft matter, 2019,15, 6044-6054
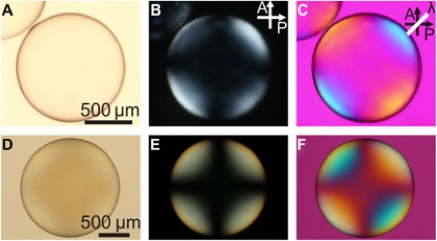
Liquid crystal elastomer shell actuators with negative order parameter, V. S. R. Jampani, R. H. Volpe, K. Reguengo de Sousa, J. Ferreira Machado, C. M. Yakacki and J. P. F. Lagerwall, Sci.adv.,DOI 10.1126/sciadv.aaw2476
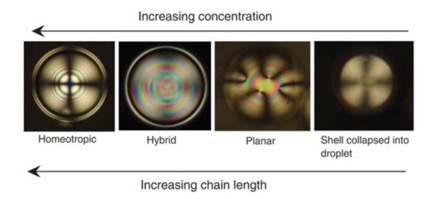
Influence of head group and chain length of surfactants using for stabilising liquid crystal shells,
Anjali Sharma and J.P.F. Lagerwall,
Liquid crystals, DOI 10.1080/02678292.2018.1509391More publications can be found here.






